And the winner is…Alphafold!
Authors: Alexandre Hocquet (Université de Lorraine and KHK Aachen), Frédéric Wieber (Université de Lorraine), Marcus Carrier (Technische Universität Berlin and KHK Aachen)Editor: Aaron Gregory (UC Riverside)
02/03/2025 | Reflections
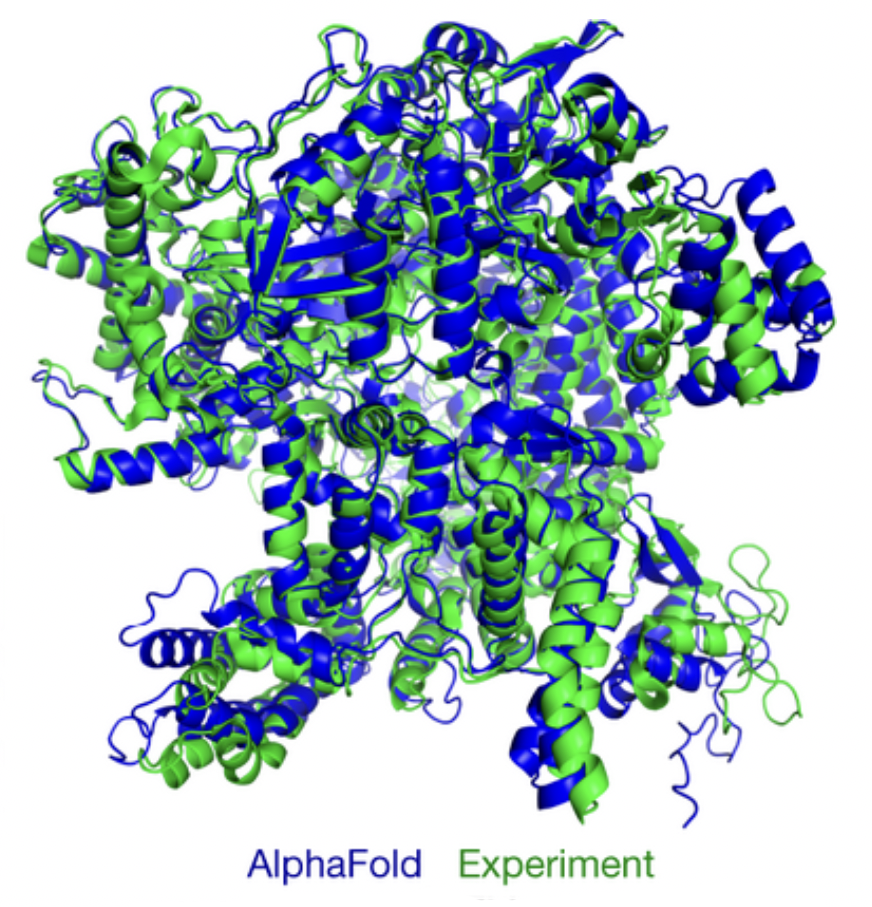
(Wikimedia Commons, 2025)
Congratulations Alphafold, recipient of the 2024 Nobel Prize in Chemistry! Although, of course, officially awarded to human beings, the most prestigious scientific award introduces artificial intelligence as a figure integral to a peculiar way of ‘doing science’, one that is based on winning contests. But what is AlphaFold, and what does its rise anticipate for the field of techno-scientific innovation?
Alphafold was developed as an artificial intelligence (AI) system by the Google subsidiary DeepMind—a tool designed to predict protein structures with a higher degree of accuracy. As the Nobel Prize press release puts it, “proteins control and drive all the chemical reactions that together are the basis of life. Proteins also function as hormones, signal substances, antibodies and building blocks of tissues” (Nobel Prize, 2024). Alphafold is now sparking significant interest in the field of structural biology and within Big Pharma. The Alphafold success story sheds light on a peculiar way of doing science: solutionist, openwashed, gamified and epistemically suited to contests.
Alphafold as techno-scientific promise
The Nobel Committee congratulates the recipients of this award for solving 'protein folding', a “fifty-year-old problem” in structural biology (Nobel Prize, 2024). Protein folding is the process by which a protein assumes its three-dimensional shape, which is crucial for its biological function. Predicting and explaining how a protein’s amino acid sequence might determine its final structure by experimental or computational methods is challenging because the number of possible shapes a protein can take is astronomically large. If early attempts at computing the mechanism of protein folding are indeed this old, it might be an exaggeration to state that the problem is now ‘solved’.
Alphafold does not explain the folding mechanism. It predicts three-dimensional structures by applying machine learning methods to compute these structures, and compares them to experimental ones (Tewilliger et al., 2024). This approach works extremely well, far better and quicker than traditional 'deterministic' computational methods that use predefined models of intramolecular physical interactions to predict a protein’s structure. This differs from AI approaches, which use data-driven learning to explore patterns. This advancement is amazing in and of itself.
Yet, there is still a long way to go before Alphafold actually leads to cancer or Alzheimer cures, as Alphafold promoters boldly claim. Their solutionist rhetoric is framed as a techno-scientific promise aimed at Big Pharma (Joly, 2010). The pharmaceutical industry has always been eager to invest in novel ways to explore potential new drugs to reduce the long and costly process of drug design. Back in the 1980s, in the booming days of computational chemistry (Hocquet & Wieber, 2017), the use of molecular modeling software packages promised the ability to design new drugs on computer screens, based on the understanding of biological targets including proteins and their molecular structures. This scientific promise, called 'rational drug design', anticipated a lucrative market based on commodified molecular modeling software packages sold to R&D departments in the pharmaceutical industry. Alphafold is now playing the same role for the same audience.
Alphafold as demo culture
During the 1970s and 1980s, the appeal for rational drug-design relied in no small amount on the shiny aesthetics of molecules as tubes and spheres catching light on the new graphics terminals of Silicon Graphics workstations (Gaboury, 2021) (the ones that would later produce the Jurassic Park dinosaurs). Today, the same shiny imagery of folding proteins accompany every Alphafold press release. Then and now, the promise serves the markets shared between Tech giants and Big Pharma. Silicon Graphics, Inc. a pioneer in graphics processing unit (GPU) computing was among the first in rational drug design. Today, it is Google doing the same with Deepmind and Alphafold.
Beyond the shiny aesthetics, protein folding is a valued and popular domain for citizen scientists participating in gamified initiatives (Cooper, 2010) including Foldit! and Fold@home. In these participatory initiatives, gamers lend their distributed computing power to fold proteins, with the aim of curing cancer and other diseases. In parallel, the Deepmind company also has corporate experience in video gaming, making a name for itself with AlphaGo (Silver et al., 2016), a machine learning program developed to play the game of Go. AlphaGo beat a human Go champion in 2016 with great fanfare in a Google-marketed performance, one that is typical of the solutionist character of the so-called 'demo culture' gaining currency in Silicon Valley (Rosental, 2021). For Deepmind, there is no doubt that protein folding and gamification are a match.
(Protein folding of Mycoplasma mycoides; Protein Data Bank, 2011)
Alphafold as an epistemic culture of contests
Prior to the current gamification process in the 1990s, the protein folding community was differently habituated to an epistemic culture of contests. The Critical Assessment of Structure Prediction (CASP) began as a biennial contest where research groups from all over the world simultaneously competed to test and demonstrate their capacities to predict protein structures selected by contest organizers. Predating Google’s interest in protein folding by several decades, CASP contests remain a major event in the predictive modeling community. Periodic puzzle solving competitions are a core component of CASP, and of a growing community that values scientific achievement through demonstrations and contests.
This way of doing science is compatible with the demo culture dear to the hearts of Silicon Valley and the academic field of computer science. Enter Deepmind: Contests are us. Deepmind brings with them an amount of computational power and dedicated personnel that only Big Tech giants can afford. No academic research group or community of citizen scientists can compete with Google when it comes to brute force the quickest solution to a given problem (and the opportunity to promote it).
Machine learning, datasets and databases
Machine learning as a solution to protein folding has a long history: the potential of machine learning within the field of protein folding was recognized beginning in the 1990s (Holley & Karplus, 1989), decades before it was actually computationally feasible, and 'before it was cool'. Computational power (in the form of GPUs) and dedicated personnel are actually what Machine Learning needs to be efficient. It is no wonder that many achievements in this field come from the corporate world, where computational and personnel resources are abundant.
But there is something else that Machine Learning needs: a good dataset on which to train its learning process. Training datasets for machine learning models are often the subject of controversies regarding their provenance. For Alphafold, there is no dispute. It is trained on the Protein Data Bank, an academic and open database of experimental protein structures established in the 1970s as a way to share structural data in the structural biology community. It was a pioneering initiative in open science (Strasser, 2019)—a bold move to imagine a database of shared crystallographic structures. Due to the openness of its data, the Protein Data Bank continues to serve as a dataset used to train algorithms for whomever has the computational resources.
Alphafold and Open Science
The true achievement of open science allows for data reuse by Google, a corporation recognized for its continued reliance upon open-source software to create new enclosures (e.g., the Android operating system building on Linux). Related controversy accompanied the announcement of the release of Alphafold 3 back in May 2024. Scientists published an open letter questioning its release due to a lack of disclosure of the source code—a disclosure delayed until very recently. It is indeed possible to use Alphafold freely as a service, but only for non-commercial purposes.
Alphafold is more than a gaming initiative or a public relations opportunity. It provides the scientific community with millions of accessible, calculated structures that actually help crystallographers determine new experimental structures. Yet, Alphafold is also very important for Google to open new market avenues, because that is the whole
point of its techno-scientific promise. Just like computational chemists sold molecular modeling software packages to Big Pharma in the 1980s, Deepmind is now funding Isomorphic Labs, a spin-off whose purpose is to deliver new potential drugs to Big Pharma.
Despite commitments to open science, this spin-off explains why Alphafold is closed for commercial applications. Its ‘non-commercial’ clause is designed to prevent competition while they expand business with Big Pharma. Like so many machine learning initiatives including chatGPT, LlaMA, or DeepSeek, the ‘open’ self-branding is a subtle game of defining or failing to disclose what is open (code, datasets, training weights) to gain or maintain a competitive advantage. It has always been a core strategy for Google and the Silicon Valley in general to benefit from an open database or source code, and then navigate the minutiae of being open or closed.
Big Tech has demonstrated a savoir-faire attitude when flooding users with tools that soon become unavoidable and indispensable. It is in this sense that AI is substantively changing how we ‘do science’. Alphafold and the rise of AI in structural biology is no exception, marking an important tension between open science initiatives and profitable enclosures of proprietary technologies. The Nobel Prize-winning Alphafold is therefore an opportunity for STS and HPS scholars to explore the techno-scientific promise and epistemic consequences of openwashed, gamified and predictive AI in protein folding and beyond.
*This text is under a CC-BY license
Published: 02/09/2025
